- new February 7, 2025
The MHDO will post a list of NDCs for Manufacturers drug(s) that hit one of the triggers during the prior calendar year as defined in 90-590 CMR Chapter 570, UNIFORM REPORTING SYSTEM FOR PRESCRIPTION DRUG PRICE DATA SETS, section 2 B 1-3.
Note: Previous years' lists can be found on the Historical Trigger NDCs page.
New Posting 2/7/25 - MHDO Posting for CY 2024 (baseline data period- 12/31/2023)For most NDC’s (drugs) we are comparing price increases to what the price was on 12/31/23 – the baseline. For some NDCs, the drug wasn’t introduced (or acquired) by the manufacturer until some point during the year – in these cases the baseline date (the date to which we compare the price after increase) is different from the last day of the previous year. The baseline date in the data sets provides the date from which we are measuring the amount of increase for a given record.
Select this link to download the Excel version of the CY 2024 report below.
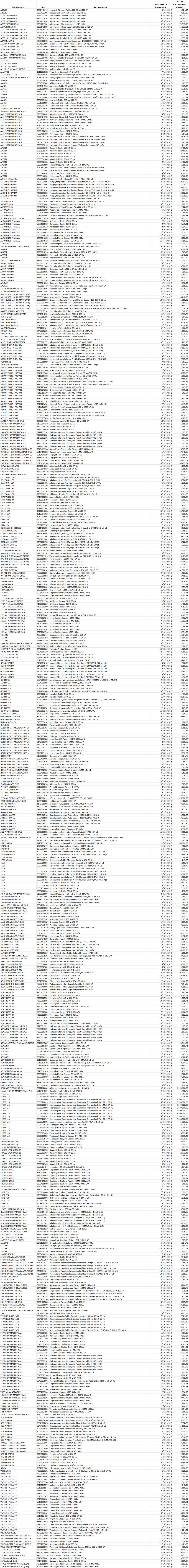
Table 1. List of brand-name drug NDCs that incurred an increase in the wholesale acquisition cost (WAC) by more than 20% per pricing unit.
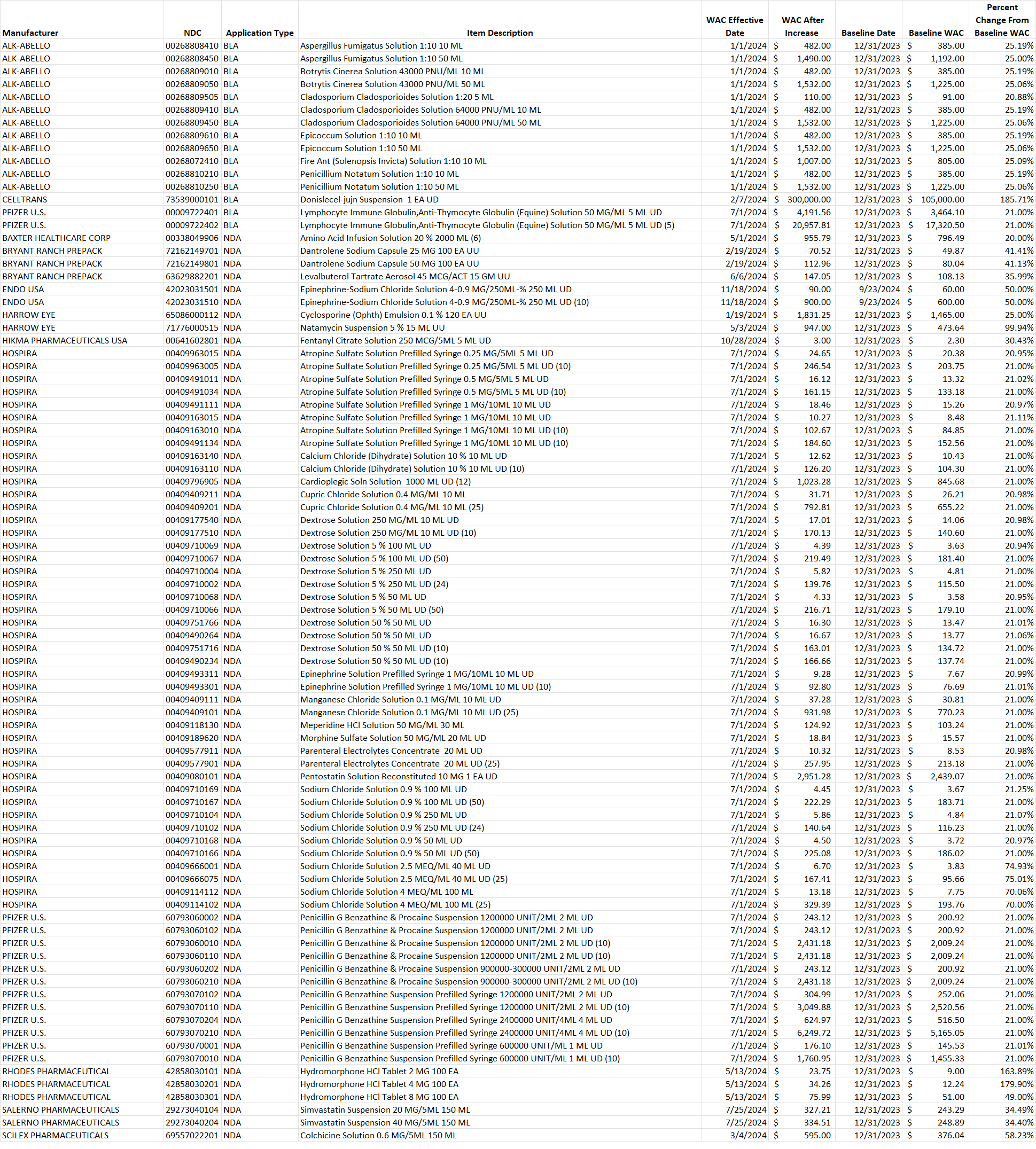
Select this link to download the Excel version of the CY 2024 report below.
Table 2. List of generic drug NDCs that costs at least $10 per pricing unit and incurred an increase in the wholesale acquisition cost (WAC) by more than 20% per pricing unit.
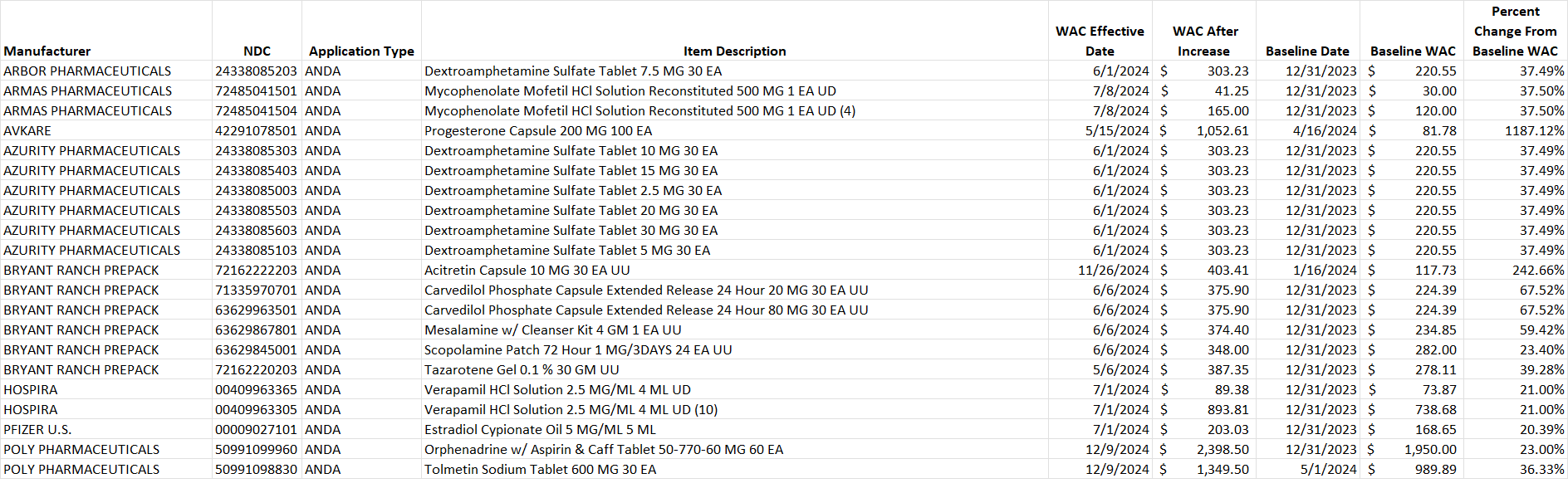
Select this link to download the Excel version of the CY 2024 report below.
Table 3. List of new prescription drugs where the wholesale acquisition cost (WAC) is greater than the amount to be considered a specialty drug under the Medicare Part D program. Note: Items listed in Table 3 may be packaged such that package contents represent more than a 30-day maximum recommended dosage. Items which fall below the Medicare Part D specialty-tier cost threshold, after application of the 30-day maximum recommended dosage to the WAC value calculated for the lowest dispensable unit of the drug product, will be removed upon notification to MHDO’s Executive Director, Karynlee Harrington (karynlee.harrington@maine.gov) and MHDO review.