
This page contains lists of NDC's for previous years. The most current lists can be found on the MHDO Trigger NDC's page.
Each year contains three tables:
Below are links that will bring you directly to each year's first table.
For most NDC’s (drugs) we are comparing price increases to what the price was on 12/31/22 – the baseline. For some NDCs, the drug wasn’t introduced (or acquired) by the manufacturer until some point during the year – in these cases the baseline date (the date to which we compare the price after increase) is different from the last day of the previous year. The baseline date in the data sets provides the date from which we are measuring the amount of increase for a given record.
Select this link to download the Excel version of the CY 2023 report below.
Table 1. List of brand-name drug NDCs that incurred an increase in the wholesale acquisition cost (WAC) by more than 20% per pricing unit.
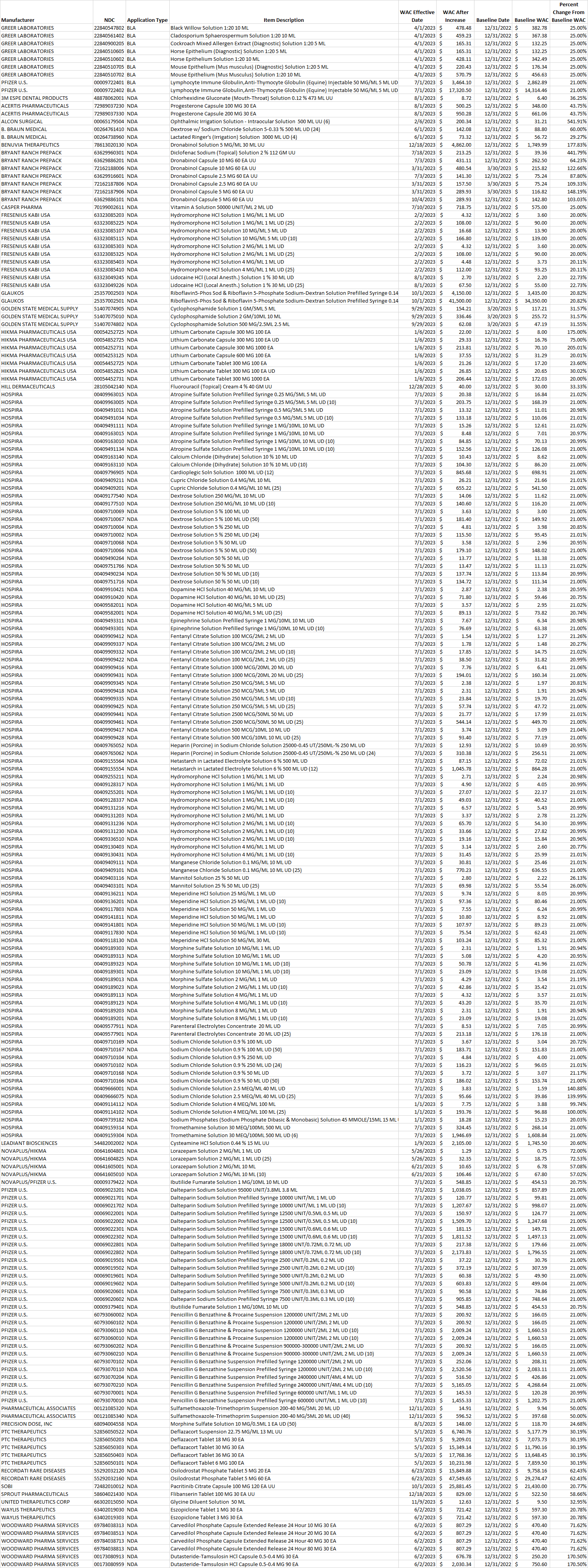
Select this link to download the Excel version of the CY 2023 report below.
Table 2. List of generic drug NDCs that costs at least $10 per pricing unit and incurred an increase in the wholesale acquisition cost (WAC) by more than 20% per pricing unit.
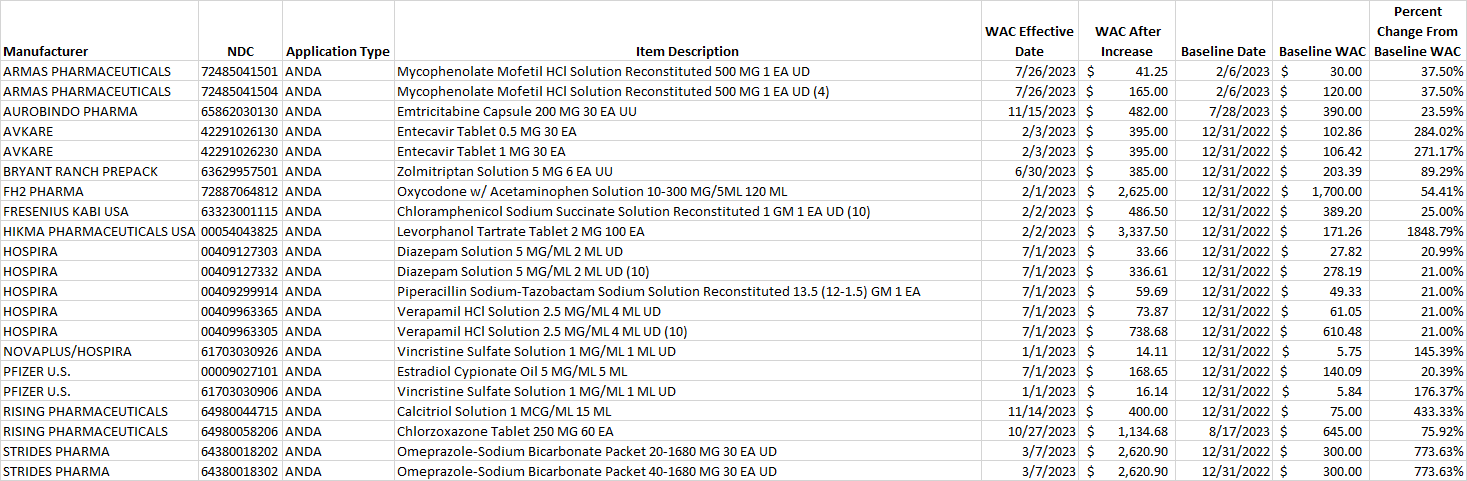
Select this link to download the Excel version of the CY 2023 report below.
Table 3. List of new prescription drugs where the wholesale acquisition cost (WAC) is greater than the amount to be considered a specialty drug under the Medicare Part D program. Note: Items listed in Table 3 may be packaged such that package contents represent more than a 30-day maximum recommended dosage. Items which fall below the Medicare Part D specialty-tier cost threshold, after application of the 30-day maximum recommended dosage to the WAC value calculated for the lowest dispensable unit of the drug product, will be removed upon notification to MHDO’s Compliance Officer, Philippe Bonneau at philippe.bonneau@maine.gov and MHDO review.

Select this link to download the Excel version of the CY 2022 report below.
Table 1. List of brand-name drug NDCs that incurred an increase in the wholesale acquisition cost (WAC) by more than 20% per pricing unit.
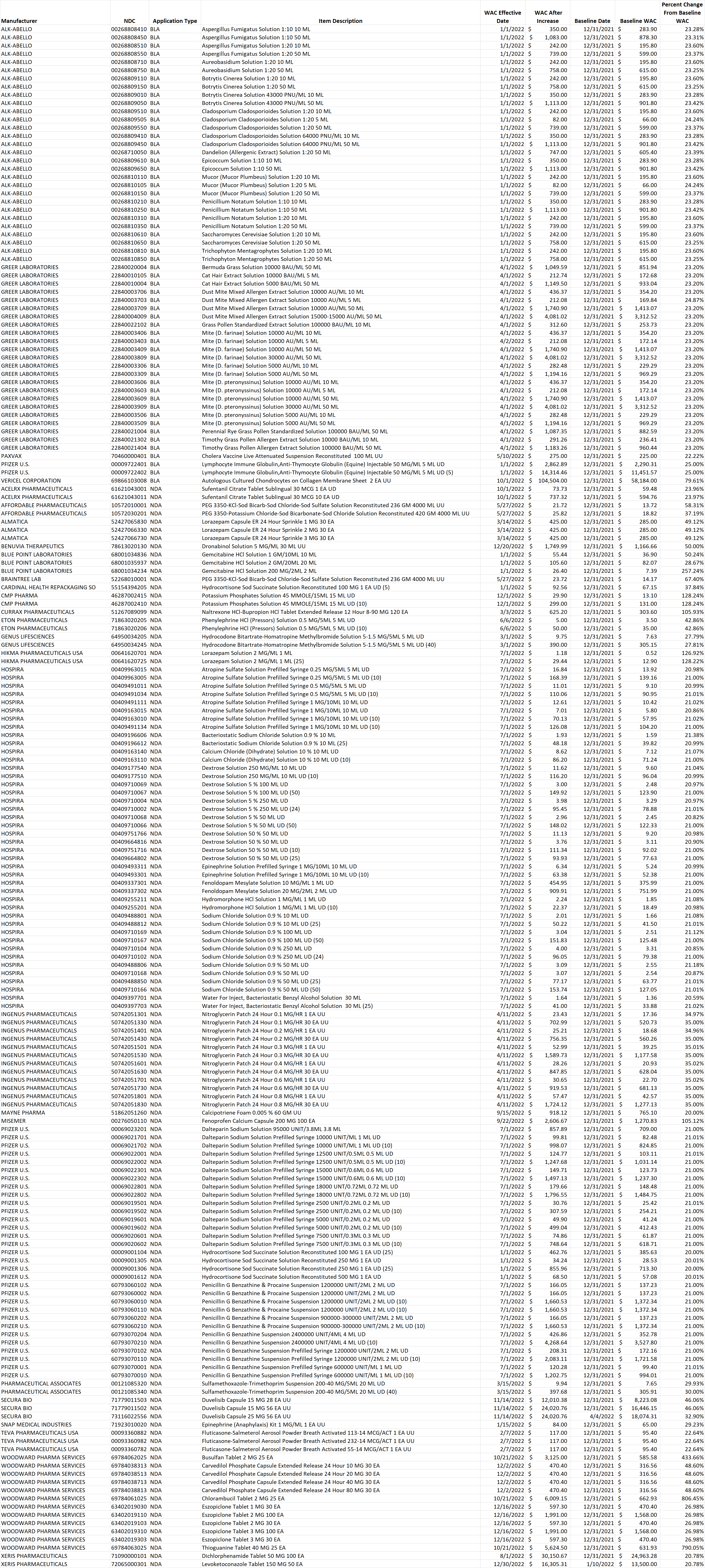
Select this link to download the Excel version of the CY 2022 report below.
Table 2. List of generic drug NDCs that costs at least $10 per pricing unit and incurred an increase in the wholesale acquisition cost (WAC) by more than 20% per pricing unit.
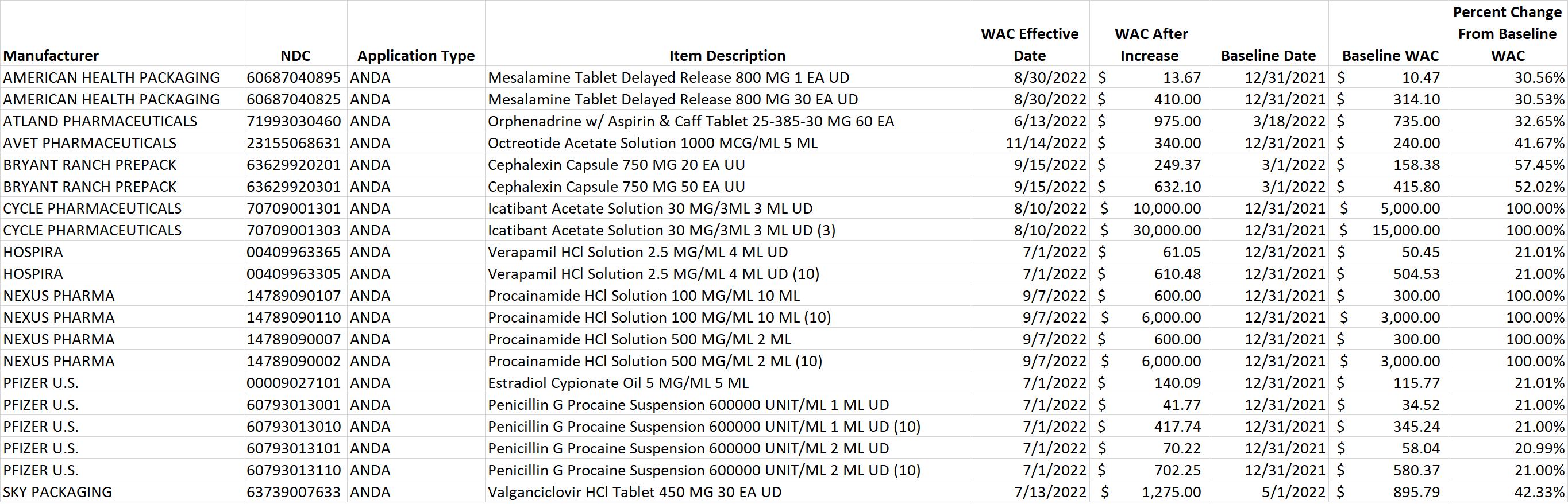
Select this link to download the Excel version of the CY 2022 report below.
Table 3. List of new prescription drugs where the wholesale acquisition cost (WAC) is greater than the amount to be considered a specialty drug under the Medicare Part D program. Note: Items listed in Table 3 may be packaged such that package contents represent more than a 30-day maximum recommended dosage. Items which fall below the Medicare Part D specialty-tier cost threshold, after application of the 30-day maximum recommended dosage to the WAC value calculated for the lowest dispensable unit of the drug product, will be removed upon notification to MHDO’s Compliance Officer, Philippe Bonneau at philippe.bonneau@maine.gov and MHDO review.
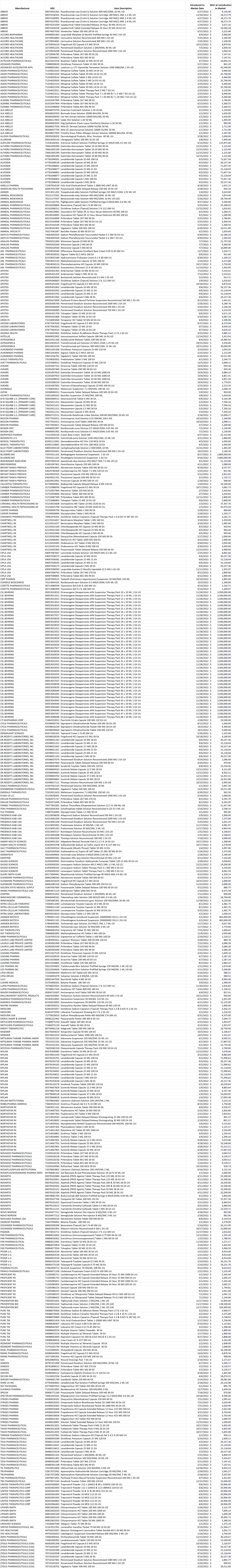
Select this link to download the Excel version of the CY 2021 report below.
Table 1. List of brand-name drug NDCs that incurred an increase in the wholesale acquisition cost (WAC) by more than 20% per pricing unit.
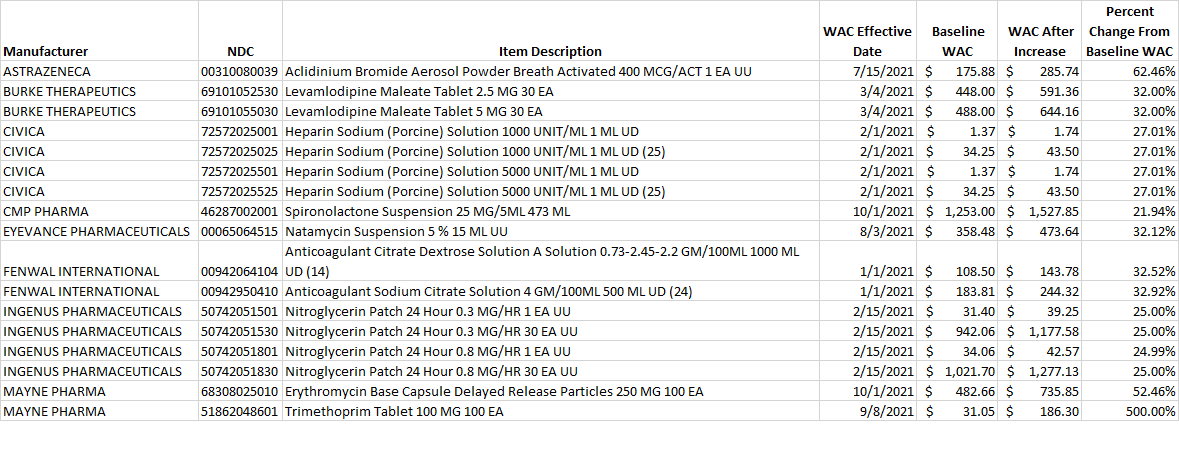
Select this link to download the Excel version of the CY 2021 report below.
Table 2. List of generic drug NDCs that costs at least $10 per pricing unit and incurred an increase in the wholesale acquisition cost (WAC) by more than 20% per pricing unit.
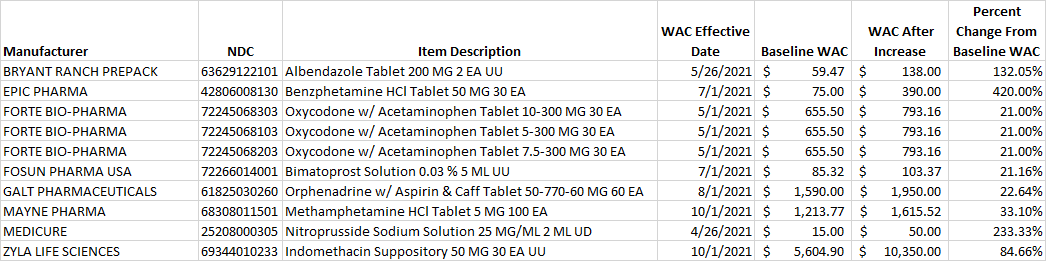
Select this link to download the Excel version of the CY 2021 report below.
Table 3. List of new prescription drugs where the wholesale acquisition cost (WAC) is greater than the amount to be considered a specialty drug under the Medicare Part D program. Note: Items listed in Table 3 may be packaged such that package contents represent more than a 30-day maximum recommended dosage. Items which fall below the Medicare Part D specialty-tier cost threshold, after application of the 30-day maximum recommended dosage to the WAC value calculated for the lowest dispensable unit of the drug product, will be removed upon notification to MHDO’s Compliance Officer, Philippe Bonneau at philippe.bonneau@maine.gov and MHDO review.
